Latest Updates in Medical Billing: CPT Code 76145 and Cybersecurity Disruptions
“The guide is intended to provide suggestions for practical clinical implementation of this code and offers key considerations, CMS current payment rates, and resources to assist ACR members,” the college said in a March 13 news update. “For those who have yet to implement this code, discuss it with your department manager/administrator.”
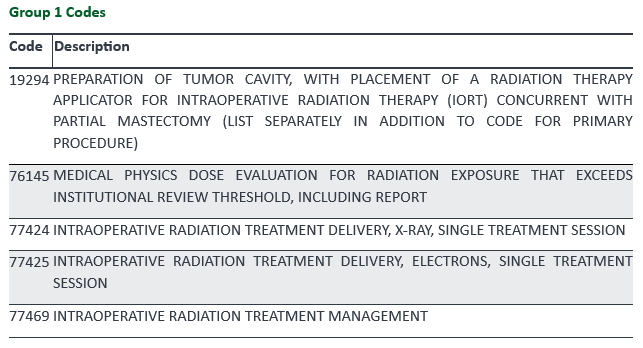
CPT® code 76145 covers the independent work of a physicist for performing a dose estimate. A physician must order the service and the request must be specific to a particular patient by the physician performing a high-dose procedure such as interoperative radiation therapy, interventional radiology/Cath lab; or as a standing order based on the facility's dose metric thresholds. The physicist calculates the total patient-specific radiation dose to the maximally exposed tissue (usually on area of skin). The final report would have a summary of the calculations and results, including the expected tissue reactions and their timing.
Palmetto GBA’s billing and coding article for Intraoperative Radiation Therapy (A56684) provides CPT/HCPCS codes that are considered medically necessary when Coverage Indications, Limitations and/or Medical Necessity are met as outlined in the Intraoperative Radiation Therapy L37779 LCD (Local Coverage Determination).
Change Healthcare Cyber-Attack: Fallout and Subsequent Responses
On February 21, 2024, Change Healthcare, a subsidiary of UnitedHealth Group (UHG), suffered what is now called the “most significant cyber-attack on the U.S. healthcare system in American history” at the hands of the Blackcat ransomware group. What makes this ransomware attack unprecedented is Change Healthcare’s role in the healthcare system – it is the main source of more than one hundred critical functions that keep the U.S. healthcare system operating. Change Healthcare’s technology manages the clinical criteria used to authorize a sizable portion of patient care and coverage; processes billions of claims; supports clinical information exchange; and processes drug prescriptions.
This cyber-attack resulted in widespread crippling of Change Healthcare’s functionality, which ultimately resulted in disruption of patient care and timely claims processing/reimbursement for hospitals, healthcare systems and medical practices. According to a survey from the American Hospital Association (AHA), more than 80 percent of hospitals state their cash flow has been affected by this breach. As of March 1, 2024, UHG created a website to serve as a resource for providing updates on their response to the cyber-attack. To mitigate the fiscal impact, UHG is offering a temporary funding assistance program, which UHG has advanced more than $2.5 billion to providers thus far. The company has also released an estimated timeline for the restoration of its services, which will be updated as the products get restored. Also in response to the cyber-attack, the U.S. Department of Health and Human Services (HHS) has announced immediate steps “that the Centers for Medicare & Medicaid Services (CMS) is taking to assist providers to continue to serve patients.” Specifically, flexibilities that have been put in place for affected providers during this type of outage:
Palmetto GBA, the part A/B MAC for Alabama, Georgia, Tennessee, North Carolina, South Carolina, Virginia and West Virginia, issued an article regarding the Change Healthcare Security, which includes the list of CMS flexibilities:
According to UHG, multiple Change Healthcare functions have been restored including pharmacy network services, the electronic payments platform so that payer implementations are proceeding, and the medical claims preparation software is back online. $14 billion in charges have been staged for processing thus far.
The HHS Office for Civil Rights (OCR) has launched an investigation into the cyber-attack. "Given the unprecedented magnitude of this cyber-attack, and in the best interest of patients and health care providers, OCR is initiating an investigation into this incident," the agency said in a statement. “OCR’s investigation of Change Healthcare and UHG will focus on whether a breach of protected health information occurred and Change Healthcare’s and UHG’s compliance with the HIPAA Rules.” UHG stated it would cooperate with the OCR investigation. Government Funding Bills Signed into Law for Fiscal Year 2024
On March 9, 2024, the President signed the Consolidation Appropriations Act, 2024 (H.R. 4366) into law. This $460 billion “minibus” package of six spending bills prevented a partial government shutdown by providing funding for fiscal year (FY) 2024 (through September 30, 2024). From a healthcare perspective, subtitle C – Medicare, contains measures which include:
The package for the six remaining appropriations bills was signed into law on March 23, 2024. Totaling $1.2 trillion, this legislation completes the omnibus government funding for FY 2024. The Further Consolidated Appropriations Act of 2024 (H.R. 2882) provides funding for the remaining federal agencies, including the Departments of Health and Human Services, Defense, Labor, Homeland Security and other priorities. The bill's package omitted several mandatory health extenders that were previously under discussion, including site-neutral and hospital price transparency provisions. Changes to the pharmacy benefit manager (PBM) industry and hospital payment reforms were also excluded due to the lack of member consensus. These legislative changes will most likely have to wait until the year end “lame duck” session. Medical Device Safety Notification
According to the FDA, Hologic, Inc., radiographic markers, BioZorb Marker and the BioZorb LP Marker, could cause serious health risks. The FDA has received reports of complications/adverse events that include pain, infection, rash, device migration, device erosion, seroma, discomfort, or other complications from feeling the device in the breast, and the need for additional medical treatment to remove the device.
The implantable radiographic markers were cleared by the FDA to be utilized to mark soft tissue sites including the breast to tag sites for future medical procedures, such as radiation planning. The BioZorb Marker and BioZorb LP Marker have two components: a resorbable plastic component that is intended to be resorbed completely by the patient's body in one year or longer, and a titanium metal component that is permanent. The BioZorb Marker and BioZorb LP Marker have not been approved by the FDA for filling space in breast tissue or improving cosmetic outcomes after procedures.
The FDA is urging radiation oncologists to closely monitor patients who have an implanted BioZorb marker for any signs of complications or adverse events. Physicians should discuss the benefits along with the potential risks of all available implantable devices or markers, and report any problem to the federal government. The FDA and Hologic, Inc. are working together to evaluate all adverse event data and address potential health risks. Hologic, Inc., did issue a device safety notification about the concern. "Patient and healthcare provider safety are our top priority, and we are committed to providing timely information to our customers that allows them to make informed treatment decisions for their patients," the company said on Feb. 28. "While we want to ensure that customers have the most up-to-date and accurate information, we have the utmost confidence in the benefits that the BioZorb marker provides to breast cancer patients and these benefits are supported by a robust body of clinical literature," it added later. Medicare Improvements to Teleradiology Requirements
The Centers for Medicare and Medicaid Services (CMS) has recently streamlined their processes regarding teleradiology services. In the past, there were many steps to be taken when a physician or non-physician practitioner (NPP) was contracted to perform teleradiology services for an organization/group located in another state. Reassignment of the contracted physician or NPP’s billing rights now takes place electronically, bringing more efficiency to the process.
The CMS-855R form that was previously used by a contracted physician or NPP to reassign their billing rights to an eligible organization/group, allowing that organization/group to submit claims and receive payment for their Medicare Part B services, has been discontinued. The separate CMS-855R reassignment form has been consolidated into the CMS-855I Medicare enrollment application for physicians and non-physician practitioners. An organization/group that contracts physicians or NPPs for teleradiology services is required to regularly submit reassignment updates. Reassignment can now be accomplished electronically by updating the reassignment section of the CMS-855I enrollment application through the Provider Enrollment, Chain and Ownership System (PECOS). More information regarding this change in the reassignment process can be found in the Consolidated CMS-8551/CMS-855R bulletin. The Medicare Program Integrity Manual, Chapter 10, Section 10.3.1.4.3 contains regulatory requirements for inter-jurisdictional reassignments. The manual states that a physician or NPP only needs to be licensed in the state where they are practicing and is not required to be licensed in the state where the image was generated (where the patient and the organization/group is located). This is different from the ACR policy that requires a physician or NPP to be licensed both in the state where they are practicing and in the state where the image was generated. Section 10.3.1.4.3 also states that the organization/group providing the service where the images are generated must enroll in both the jurisdiction where they are physically located, and in the jurisdiction where the physician or NPP providing interpretations is practicing. When submitting for enrollment in the jurisdiction where the physician or NPP providing interpretations is practicing, the organization/group providing the services where the images are generated is required to use the physician or NPP’s practice location in the practice location information section of the CMS-855B Medicare enrollment application form for clinics/group practices and other suppliers. The organization/group does not have to be licensed to provide services in the state where the physician providing interpretations is practicing. FES-PET Site Directory Created by the Society of Nuclear Medicine and Molecular Imaging
Fluoroestradiol F18 (FES) is known in the United States as Cerianna™. This radiopharmaceutical is approved by the U.S. Food and Drug Administration (FDA) as a radioactive diagnostic agent that is indicated for use with positron emission tomography (PET) imaging for the detection of estrogen receptor (ER)-positive lesions as an alternative to biopsy in patients with recurrent or metastatic breast cancer.
The Society of Nuclear Medicine and Molecular Imaging (SNMI) has compiled a list of U.S. sites performing FES-PET (Cerianna™) imaging. This resource is available for both physicians and patients seeking this type of imaging. Any site that provides FES-PET (Cerianna™) imaging can use the FES Directory Form, or email Karin Brough at [email protected], to be added to the list. According to breastcancer.org, a leading online resource for information about breast cancer and health, approximately 80% of primary breast cancers are ER+. Despite a favorable prognosis and effective therapies available for ER+ breast cancer, metastatic breast cancer from an ER+ primary tumor is responsible for the majority of breast cancer related deaths. While breast tissue itself is relatively easy and safe to biopsy, treating metastatic breast cancer is challenging. Lesion(s) may be in sites that are difficult to biopsy, such as the brain or bone. In some cases, when breast cancer has spread to distant organs and multiple sites, biopsy may not be feasible. The standard of care is typically to biopsy the metastatic site and, assuming the biology of all lesions is the same, determine treatment. However, estrogen receptor discordance can occur with ER+ metastatic breast cancer. ER expression can vary between lesions and has the potential to change over time. Metastatic lesions may become ER-, altering treatment decisions. FES-PET (Cerianna™) imaging enables a non-invasive, comprehensive assessment of whole-body ER+ lesion status. The role of FES is to give physicians a comprehensive assessment of the biology across multiple metastatic lesions, rather than basing clinical decision making on a single biopsy site. This assessment can then help guide therapeutic decision making. The creation of a site directory by the Society of Nuclear Medicine and Molecular Imaging is a welcome resource for patients and physicians in need of FES-PET (Cerianna™) imaging. More information on Cerianna™ and its role as “the only imaging agent that detects whole-body ER+ lesion status” can be found on the manufacturers website, GE HealthCare.
All rights reserved. No part of this newsletter may be reproduced in any form whatsoever without written permission from the publisher. This newsletter may reflect coding information from the 2024 Physician’s Current Procedural Terminology (CPT® Manual). CPT is a registered trademark of the American Medical Association. CPT® five-digit codes, nomenclature and other data are copyright 2023 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in CPT®. This product should not be considered a substitute for the codes, cross-references and exclusions located in the CPT® Manual. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein.
Comments are closed.
|
Categories
All
Archives
July 2024
|
|
DO YOU HAVE A QUESTION?
WE HAVE AN ANSWER. Office: 512.583.2000
Fax: 512.583.2002 |
FOLLOW THE LATEST INDUSTRY TRENDS
|
CELEBRATING 25 YEARS
|
@2024 Revenue Cycle Coding Strategies. All rights reserved.